- Home
- Businesses
-
-
-
Industrial NanoTech Businesses
-
What’s New
Many pandemic-driven changes to cancer clinical trials should remain
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Immunotherapy May Be Effective for Subset of Patients With Prostate Cancer
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Gap in Breast Cancer Survival for Black, White Patients Shrinks, But Not by Enough
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
-
-
-
-
-
- Brands
-
-
-
Industrial NanoTech Brands
-
What’s New
Many pandemic-driven changes to cancer clinical trials should remain
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Immunotherapy May Be Effective for Subset of Patients With Prostate Cancer
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Gap in Breast Cancer Survival for Black, White Patients Shrinks, But Not by Enough
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
-
-
-
-
- Research & Innovation
-
-
-
Industrial NanoTech Research & Innovation
-
What’s New
Many pandemic-driven changes to cancer clinical trials should remain
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Immunotherapy May Be Effective for Subset of Patients With Prostate Cancer
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Gap in Breast Cancer Survival for Black, White Patients Shrinks, But Not by Enough
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
-
-
-
- Self Reporting TechnologiesLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Advanced Thermal InsulationLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Corrosion Prevention TechnologiesLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Clean Energy GenerationLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Workplace SafetyLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Nurturing ScientistsLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
-
-
-
- Industries
-
-
-
Industrial NanoTech Industries
-
What’s New
Many pandemic-driven changes to cancer clinical trials should remain
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Immunotherapy May Be Effective for Subset of Patients With Prostate Cancer
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Gap in Breast Cancer Survival for Black, White Patients Shrinks, But Not by Enough
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
-
-
-
-
- Careers
-
-
-
Industrial NanoTech Careers
-
What’s New
Many pandemic-driven changes to cancer clinical trials should remain
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Immunotherapy May Be Effective for Subset of Patients With Prostate Cancer
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Gap in Breast Cancer Survival for Black, White Patients Shrinks, But Not by Enough
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
-
-
-
- JobsLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Benefits & RewardsLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Life At Industrial NanotechLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- How We HireLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- StudentsLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
-
-
-
- Newsroom
-
-
-
Industrial NanoTech Newsroom
-
What’s New
Many pandemic-driven changes to cancer clinical trials should remain
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Immunotherapy May Be Effective for Subset of Patients With Prostate Cancer
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Gap in Breast Cancer Survival for Black, White Patients Shrinks, But Not by Enough
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
-
-
-
- NewsroomLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Media ContactsLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Media CoverageLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Social MediaLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- VideosLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
-
-
-
- Investor Relations
-
-
-
Industrial NanoTech Investor Relations
-
What’s New
Many pandemic-driven changes to cancer clinical trials should remain
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Immunotherapy May Be Effective for Subset of Patients With Prostate Cancer
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Gap in Breast Cancer Survival for Black, White Patients Shrinks, But Not by Enough
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
-
-
-
- Investor RelationsLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- NewsLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Events and presentationsLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Stock InformationLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- SEC FilingsLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- GovernanceLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
-
-
-
- Our Company
-
-
-
Industrial NanoTech Our Company
-
What’s New
Many pandemic-driven changes to cancer clinical trials should remain
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Immunotherapy May Be Effective for Subset of Patients With Prostate Cancer
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Gap in Breast Cancer Survival for Black, White Patients Shrinks, But Not by Enough
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
-
-
-
- Company HistoryLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Leadership TeamLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- What We Stand ForLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Quality PolicyLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Community ManagementLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Awards & RecognitionLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
-
-
-
- Contact Us
-
-
-
Industrial NanoTech Contact Us
-
-
-
-
- Headquarter locationLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Job searchLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Submit RFPLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
-
-
-
- Become a Distributor
Industrial NanoTech
Navigation
- Home
- Businesses
-
-
-
Industrial NanoTech Businesses
-
What’s New
Many pandemic-driven changes to cancer clinical trials should remain
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Immunotherapy May Be Effective for Subset of Patients With Prostate Cancer
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Gap in Breast Cancer Survival for Black, White Patients Shrinks, But Not by Enough
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
-
-
-
-
-
- Brands
-
-
-
Industrial NanoTech Brands
-
What’s New
Many pandemic-driven changes to cancer clinical trials should remain
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Immunotherapy May Be Effective for Subset of Patients With Prostate Cancer
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Gap in Breast Cancer Survival for Black, White Patients Shrinks, But Not by Enough
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
-
-
-
-
- Research & Innovation
-
-
-
Industrial NanoTech Research & Innovation
-
What’s New
Many pandemic-driven changes to cancer clinical trials should remain
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Immunotherapy May Be Effective for Subset of Patients With Prostate Cancer
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Gap in Breast Cancer Survival for Black, White Patients Shrinks, But Not by Enough
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
-
-
-
- Self Reporting TechnologiesLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Advanced Thermal InsulationLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Corrosion Prevention TechnologiesLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Clean Energy GenerationLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Workplace SafetyLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Nurturing ScientistsLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
-
-
-
- Industries
-
-
-
Industrial NanoTech Industries
-
What’s New
Many pandemic-driven changes to cancer clinical trials should remain
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Immunotherapy May Be Effective for Subset of Patients With Prostate Cancer
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Gap in Breast Cancer Survival for Black, White Patients Shrinks, But Not by Enough
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
-
-
-
-
- Careers
-
-
-
Industrial NanoTech Careers
-
What’s New
Many pandemic-driven changes to cancer clinical trials should remain
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Immunotherapy May Be Effective for Subset of Patients With Prostate Cancer
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Gap in Breast Cancer Survival for Black, White Patients Shrinks, But Not by Enough
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
-
-
-
- JobsLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Benefits & RewardsLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Life At Industrial NanotechLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- How We HireLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- StudentsLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
-
-
-
- Newsroom
-
-
-
Industrial NanoTech Newsroom
-
What’s New
Many pandemic-driven changes to cancer clinical trials should remain
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Immunotherapy May Be Effective for Subset of Patients With Prostate Cancer
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Gap in Breast Cancer Survival for Black, White Patients Shrinks, But Not by Enough
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
-
-
-
- NewsroomLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Media ContactsLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Media CoverageLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Social MediaLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- VideosLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
-
-
-
- Investor Relations
-
-
-
Industrial NanoTech Investor Relations
-
What’s New
Many pandemic-driven changes to cancer clinical trials should remain
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Immunotherapy May Be Effective for Subset of Patients With Prostate Cancer
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Gap in Breast Cancer Survival for Black, White Patients Shrinks, But Not by Enough
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
-
-
-
- Investor RelationsLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- NewsLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Events and presentationsLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Stock InformationLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- SEC FilingsLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- GovernanceLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
-
-
-
- Our Company
-
-
-
Industrial NanoTech Our Company
-
What’s New
Many pandemic-driven changes to cancer clinical trials should remain
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Immunotherapy May Be Effective for Subset of Patients With Prostate Cancer
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
Gap in Breast Cancer Survival for Black, White Patients Shrinks, But Not by Enough
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been…
-
-
-
- Company HistoryLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Leadership TeamLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- What We Stand ForLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Quality PolicyLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Community ManagementLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Awards & RecognitionLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
-
-
-
- Contact Us
-
-
-
Industrial NanoTech Contact Us
-
-
-
-
- Headquarter locationLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Job searchLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
- Submit RFPLorem ipsum is a placeholder text commonly used to demonstrate the visual form of a document or a typeface without relying on meaningful content.
-
-
-
- Become a Distributor
Industrial NanoTech
Search
Frequently Asked Questions
Industrial NanoTech
Contacts
Address
Naples, FL 34109
Industrial NanoTech
<span data-metadata="“><span data-buffer="“>Social Network
Business
Subsurface Thermal Insulation
Thermal Insulation for Buildings
Insulation products have developed significantly with technological advances. Legislation has acted as the catalyst for development, from the basic requirements under the Building Regulations Part L, to compliance with government carbon reduction targets, driven through advanced programmes such as the Code for Sustainable Homes and BREEAM.
Insulation products vary in terms of colour, surface finish and texture, core composition and, importantly, performance. The specification of materials that insulate is a science-based decision, but a successful specification relies on the specifier understanding not only the mathematical performance, but the peripheral factors that can influence the final installation.
Specification of insulation products is often based upon the minimum requirement of the Building Regulations AD (Approved Document) Part L and their relationship with manufacturers performance data, and it has been suggested that legislation is driving the production of a range of products that ‘just work’, presenting little apparent difference between them.
In order to specify insulation correctly however, the specifier needs to understand the reasons why it works, and apply the correct technology to any given construction detail. In understanding more fully the processes that make insulation work, and indeed the factors that stop it from working, specifiers will be in a far stronger position to specify the correct material for the correct application.
The installed performance of an insulation product is reliant upon not only performance characteristics and the adherence of contractors to manufacturers and general best practice workmanship requirements, but also the suitability of the insulant specified to its installed location.
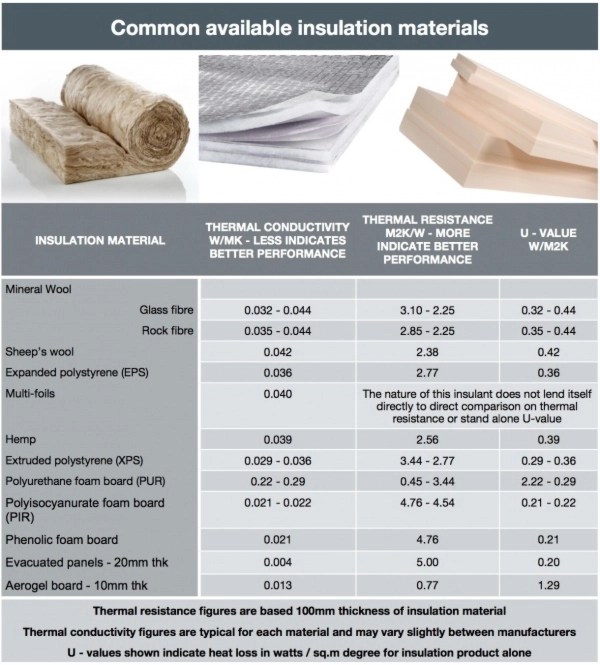
How insulation works
Insulation products are designed to frustrate the transfer of heat across the material itself. There are three methods of heat transfer: radiation, conduction and convection.
Radiation
Any object whose temperature is higher than the surfaces that surround it will lose energy as a net radiant exchange. Radiant heat can only travel in straight lines. Introduce a solid object between points A and B, and they will no longer directly exchange radiant heat. Radiation is the only heat transfer mechanism that crosses vacuums.
Conduction
Conduction is reliant upon physical contact. If there is no contact, conduction cannot take place. Contact between two substances of different temperature results in a heat exchange from the higher temperature to the lower temperature substance. The greater the temperature differential, the faster the heat exchange.
Convection
Convection is the transfer of energy via fluids (gases and liquids). It is this method that plays the greatest role in the liberation and transfer of heat in buildings. The most common propagation of this effect is from solid to gas, i.e. object to air, and then back again, typically as the air meets with the external building fabric.
The process is actually initiated by an energy transfer due to conduction, and is complicated by the level of water vapour that is supported by the air. The water molecules store heat given to them through conduction from warm surfaces. The water vapour and the air cannot be separated as gases. They will only part company when the saturated vapour pressure is reached, i.e. the quantity of water (albeit in vapour form) exceeds the level of heat available to maintain it as a gas (vapour), and therefore it condenses.
Condensation causes this latent heat to be released; the temperature to water vapour ratio alters, and once it has altered far enough the process will start again. The world’s weather systems follow a very similar cycle.
If air could be kept still and dry it would perform as a highly efficient insulant. However, if air is heated, its molecular structure expands and becomes less dense relative to the air surrounding it, and so rises. As it progresses further from the heat source, it begins to cool. The molecules contract and increase in density and sink back down. Air molecules are in a constant state of flux, dependent on the ambient temperature, and interference from any point, or background heat sources.
This process of heat transfer ‘convection’ is complicated by the fact that air will cool at a rate dependent upon the amount of water vapour saturation. The greater the saturation, the slower the cooling.
Independent Coatings Sales Repsentative

Let’s Talk
Contact Us
Full Product Catalog


























